Abstract
Keywords
Allogeneic transplantation; endothelial damage; biomarkers.
Background
Endothelial damage is associated with severe complications and increased risk of death after allogeneic hematopoietic cell transplantation (AlloHCT). The recently developed Endothelial Activation and Stress Index (EASIX) is a prognostic tool that uses clinical lab values and has been shown to predict non-relapse mortality (NRM) and overall survival (OS) at onset of acute graft versus host disease (aGVHD) in reduced intensity (RIC) alloHCT (Luft, Lancet Haematol 2017). We hypothesized that EASIX may be valuable for more broadly predicting aGVHD, NRM and OS after AlloHCT, beyond time of onset of aGVHD.
Design
We evaluated 152 adult patients who received an unmodified RIC AlloHCT from a related or unrelated donor with uniform GVHD prophylaxis of sirolimus/tacrolimus and low-dose MTX for treatment of lymphoid malignancies, between April 2008 and May 2017. The EASIX formula (LDH*Creatinine/platelet counts) was calculated at multiple timepoints (pre-HCT, day 30, day 100, onset TMA and aGVHD). For all EASIX assessments post-HCT, a landmark analysis was conducted at the given timepoint A log transformation using base 2 (log2) was applied to all EASIX variables to reduce skew. A one-unit increase in log2 EASIX is associated with a doubling (one-fold increase) of EASIX on the original scale. Kaplan-Meier, cumulative incidence, and cox modeling (cause specific for NRM and aGVHD) were used to evaluate EASIX as it relates to outcomes of interest. Relapse and death or relapse were considered competing risks for NRM and aGVHD respectively.
Results
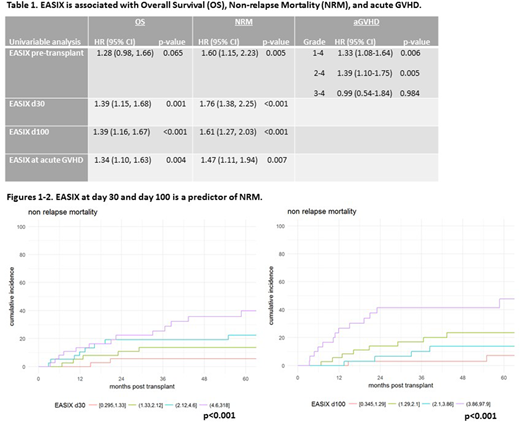
The median age at transplant was 54 years (range 23-78), 70% were males, a majority had non-Hodgkin lymphoma (68%), and most had sensitive disease at time of HCT (CR=56%; PR=33%). All patients, except two, received peripheral blood stem cells. Sixty-three patients had an HLA-identical related donor, while the remaining 89 had an unrelated donor transplant (HLA-matched in 75 patients, and HLA-mismatched in 14 patients). HCT-CI was 0 in 49 patients, 1-2 in 41 and ≥ 3 in 62 patients. With a median follow-up in surviving patients of 5.4 years (range, 0.8-10), the 1 and 3 years OS rate was 84.2% (95% CI, 77.3-89.1) and 67.9% (95% CI, 59.6-74.8), respectively. The NRM rate at 1 and 3 years was 7.9% (95% CI, 4.3-12.9) and 16.4% (95% CI, 10.9-22.8), respectively. The 1-year cumulative incidences of grades 1-4, 2-4 and 3-4 aGVHD were 56.6% (95% CI, 48.3-64.1), 42.1% (95% CI, 34.2-49.8) and 7.9% (95% CI, 4.3-12.9), respectively. Post-HCT thrombotic microangiopathy was only observed in 13 patients, representing too few events for EASIX analysis. As expected, HCT-CI was significantly associated with both OS and NRM. Pre-HCT EASIX was significantly associated with increased NRM (HR=1.60 [95% CI, 1.15-2.23], p=0.005) and aGVHD grade 1-4 and 2-4 (HR=1.33 [95% CI, 1.08-1.64], p=0.006 and HR=1.39 [95% CI, 1.10-1.75], p=0.005; respectively), but not OS or grade 3-4 aGVHD (Table 1). EASIX at day 30 and day 100 was significantly associated with both OS and NRM (Figures 1-4). Furthermore, confirming the results of Luft, EASIX calculated at onset of any grade aGVHD was significantly associated with OS (HR=1.34 [1.10-1.63], p=0.004) and NRM (HR=1.47 1.11-1.94], p=0.007). Finally, there was no correlation between HCT-CI and EASIX score.
Conclusions
We conclude that the EASIX formula, calculated at various timepoints pre and post AlloHCT, is significantly associated with NRM and OS. Pre-HCT EASIX also predicts risk of aGVHD, confirming prior results, EASIX at onset of acute GVHD is a predictor of NRM and OS in adult recipients RIC AlloHCT. EASIX provides an independent and easily accessible tool to predict important AlloHCT outcomes that can be used in addition to HCT-CI to better risk stratify patients.
Sauter:Juno Therapeutics: Consultancy, Research Funding; Sanofi-Genzyme: Consultancy, Research Funding; Spectrum Pharmaceuticals: Consultancy; Novartis: Consultancy; Precision Biosciences: Consultancy; Kite: Consultancy. Perales:Incyte: Membership on an entity's Board of Directors or advisory committees, Other: Personal fees and Clinical trial support; Merck: Other: Personal fees; Takeda: Other: Personal fees; Abbvie: Other: Personal fees; Novartis: Other: Personal fees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal